Projects of the Research Unit FOR 2858
Overall description of the Research Unit
Role of translocator protein (18 kDa) (TSPO) as a diagnostic and therapeutic target in the nervous system
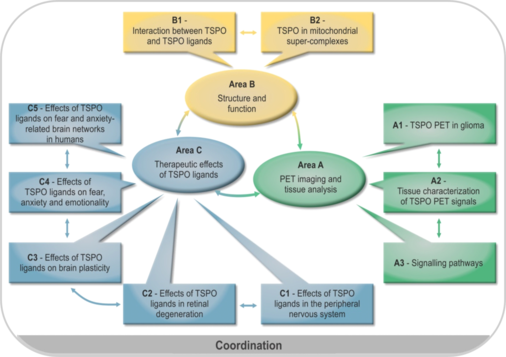
TSPO is a ubiquitous mitochondrial protein and is involved in numerous biological functions. Although TSPO is particularly abundant in steroid producing tissues it can be found substantially also in the brain, liver, heart and the immune system. Within the cell, TSPO is predominantly located in mitochondria, specifically in the outer mitochondrial membrane. It belongs to a multimeric complex and is associated with other proteins.The pleiotropic actions of TSPO render this protein an interesting target in the nervous system. Therefore, the planned research group aims to characterize the role of TSPO as a diagnostic and therapeutic target from structural biology over neurology to psychiatry and to delineate the potential of TSPO ligands as a novel therapeutic approach within the nervous system. To reach this goal, scientists from the Universities of Regensburg and Munich as well as experts in TSPO research from Göttingen and Paris, who have already worked together in the field, will take part in this endeavor. The topic will be addressed in three research areas. Area A will delineate the diagnostic potential of TSPO imaging by means of positron emission tomography (PET) in brain tumors in vivo and will enable the complementary characterization of brain tissue in vitro to delineate the neurobiological characteristics underlying TSPO PET labeling in the human brain. Area B will provide information on the structure and function of the TSPO molecule in response to TSPO ligands and within its multimeric complex by biophysical methods in order to unravel further molecular mechanisms of TSPO signaling and to give further input for TSPO ligand drug development. Area C will address the role of TSPO for neurodegeneration within the peripheral nervous system, the retina and the brain using newly established animal models and PET imaging. Moreover, the neuroregenerative potential of TSPO ligands will be investigated thereby providing information on the consequences of structure-activity relationship of TSPO ligands in vivo. In addition, the role of TSPO for behavior such as anxiety, fear and stress in relation to the modulating effects of TSPO ligands will be studied in respective animal models including PET labeling in relation to neurosteroidogenesis and spine plasticity. There will be a translation to humans by means of neuroimaging based on cognitive neuroscience approaches. It will be of interest to define brain networks specifically targeted by TSPO ligands. The findings within area C can ultimately be translated to neurodegenerative and psychiatric disorders. In conclusion, the planned research group aims to put forward TSPO as a diagnostic and therapeutic target in the nervous system in a complimentary translational approach. The coordination proposal describes measures and funding for coordination of the research unit.
A1: TSPO PET in glioma
Nathalie Albert, Peter Bartenstein, Jörg-Christian Tonn, Munich
TSPO PET labeling in gliomas as a diagnostic marker in the human brain
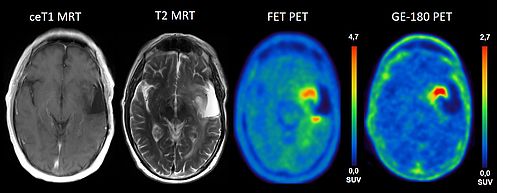
TSPO is overexpressed in primary brain tumors and has been shown to correlate with the malignancy of the tumor cells. Previous work has shown that the PET signal of gliomas in TSPO PET and amino acid PET ([18F]FET) are different and also differ from contrast enhancement in MRI. So far, it is not known which tumor features are reflected by the different imaging modalities and whether this has an impact on the clinical outcome and prognosis of patients.
This project aims to delineate the neurobiology underlying TSPO overexpression in the human brain together with projects A2 and A3. Moreover, it will investigate whether combined TSPO PET and FET PET imaging can delineate tumor borders more reliably than structural MRI and whether an increased TSPO PET signal is associated with aggressive tumor features in heterogeneous gliomas.
We plan in work package 1 a prospective clinical evaluation of three patient populations (suspected glioblastoma, glioma without contrast enhancement on MRI and progressive/recurrent glioma) prior to stereotactic biopsy or surgical resection. All patients will undergo PET imaging with the high affinity TSPO ligand [18F]GE-180, FET-PET and MRI prior to the neurosurgical intervention. Tumor resection and serial stereotactic biopsies will be performed using an image guided neuronavigation system. The exact localizations of tissue samples will be assigned to PET and MRI data, which enables a precise spatial correlation of PET information with histopathological findings and pathway analysis (project A2/A3). Recruitment phase of the study will be 18 months with follow-up of another 18 months.
In the first half of the funding period the major aim of work package 2 will be the development of an easy to handle software tool for the voxelwise visualization of increased amino acid transport, differences in FET-kinetics, [18F]GE-180 accumulation and overlap zones between blood-brain-barrier disruption, increased amino acid transport and microglia activation.
The extended set of data, which will be acquired within work package 1, 3 and project A2 (elaborated imaging, clinical data and histological correlation), provides a unique opportunity to apply automated image analysis and machine learning. The goal of this work package in the second half of the funding period is to establish a link between image features with the location and time of local relapse and ultimately the overall survival. Part of work package 2 will also be the establishment of TSPO PET imaging at the University Hospital Regensburg.
Work package 3 deals with the set up of a clinical data base for systematic integration of clinical, imaging and neuropathological data. All data retrieved from clinical workup, in particular from clinical follow-up during the disease course, initial and follow-up imaging and neuropathological analysis (histology and molecular profile of each sample) will be fully registered in the dedicated databank for further analysis.
A2: Tissue characterization of TSPO PET signals
Markus J. Riemenschneider, Regensburg
Histological and molecular characterization of TSPO labeling in human brain tissue
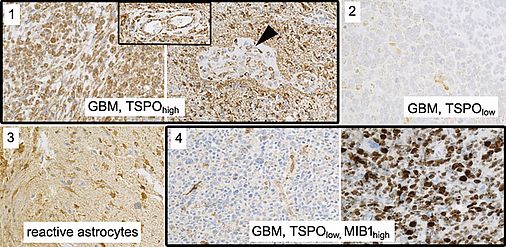
TSPO has been suggested as a promising novel target for brain tumor imaging. Particularly in glioblastoma, the most aggressive glial brain tumor, consistently high levels of TSPO expression have been observed, while TSPO expression is low in non-neoplastic brain tissue. Slightly higher uptake has also been reported in the peripheral regions of the tumor suggesting that TSPO PET imaging can identify the proliferative and infiltrative components of gliomas. Clarity of the interpretation of a TSPO signal, however, may be affected by the heterogeneity of cell populations contained in the tumor (glial tumor cells, reactive astrocytes, endothelial cells, glioma-associated microglial cells/macrophages). It is unclear to what extent these contaminating cell populations contribute to the TSPO signal in the individual lesion and thus might mislead interpretation. Also, TSPO expression is not exclusively upregulated in neoplastic conditions but also in the brain at sites of injury, inflammation as well as ischemia, demyelination and neurodegeneration.
We thus aim to perform a careful dissection of TSPO expression on a histological and cellular level in human glioma biopsy samples to confer clarity concerning the interpretation and the relevance of TSPO PET enrichment patterns. We will comprehensively compare the tissue qualities and cell populations contained in samples from TSPOhigh and TSPOlow labeled regions. We will employ NGS (Next Generation Sequencing) analyses (DNA panel sequencing and RNAseq) to assess the underlying genomic and transcriptional alterations on a broad scale. We will also perform in situ analyses (immunohistochemistry) to track molecular alterations within the histological and cellular background. We will clarify the contribution of epigenetic alterations on TSPO inactivation and assess TSPO-related signaling pathway activation to foster our notion of TSPO biology.
The assessment of TSPO expression and regulation in close connection with the underlying histological and molecular information as well as available clinical data will constitute an important step towards the interpretation of TSPO labeling in the human brain and a better understanding of TSPO function in gliomas.
A3: Signalling pathways
Philipp Beckhove, Peter Hau, Regensburg
The role of TSPO in T cell immune control of glioblastoma
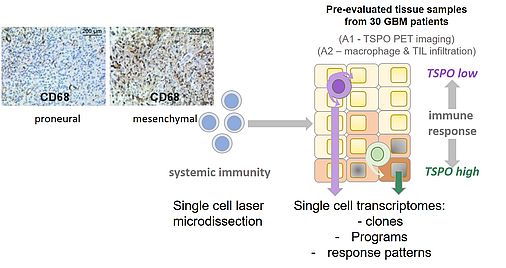
Glioblastoma (GB) is the most malignant and common type of primary brain tumor with a median survival of less than 21 months. Apart from molecular alterations, the pathogenesis of GB is largely dependent on immune escape mechanisms. However, immunotherapies yet demonstrated limited efficacy – most likely due to the heterogeneous nature of GB and adaptive resistance mechanisms, which develop throughout treatment.
The ineffectiveness of current immunotherapy for GB suggests the existence of yet undetected mechanisms of immune resistance and clearly underlines the need for identifying novel therapeutic targets.
TSPO is an 18 kDa protein which is potentially involved in numerous biological functions in GB. Although TSPO is particularly abundant in steroid producing tissues, it is also expressed in the brain and the immune system. Due to its up-regulated expression compared to normal brain, positive correlation with malignancy, and inverse correlation with clinical outcome, TSPO is increasingly used as a prognostic biomarker for GB. Preliminary data also suggest a role of TSPO in the regulation of apoptosis, although mechanistic aspects remain unclear. TSPO expression in the tumor itself also positively relates to immune infiltration. However, a role of TSPO in regulating immune resistance of human tumors and specifically GB has not been fully explored so far.
During the first funding period, we focused on three major goals related to human GB. First, we intended to clarify whether TSPO mediates resistance of GB cells against cytotoxic T-cell attack. Second, we assessed molecular mechanisms of an immune modulatory role and third, we investigated how TSPO expression is regulated in GB tumors.
We found that TSPO expression in BTICs is induced upon contact with tumor antigen-reactive T-cells and T-cell-derived soluble factors. As the inhibition of IFNγ and TNFα or their receptors abrogated TSPO upregulation in T-cell co-cultures, we concluded that T-cell-derived IFNγ and TNFα are the main inducers of TSPO in GB. Our data thereby support the hypothesis that cytotoxic T-cell responses are important drivers of TSPO expression in BTIC. We next studied whether TSPO mediates immune resistance in GB cells. To this end, we observed that genetic TSPO downregulation sensitized GB cell lines and BTICs against T-cell mediated cytotoxicity in both allogeneic and autologous settings and impaired migratory capacities. Moreover, mechanistic studies showed that TSPO protects BTICs selectively against TNF-related apoptosis inducing ligand (TRAIL)-induced apoptosis by regulating both intrinsic and extrinsic apoptotic pathways. In addition to genetic manipulation of TSPO expression, we regulated TSPO activity by applying synthetic ligands. The ligands consistently increased the pro-apoptotic effects of TRAIL and impaired mitochondrial activity.
Our finding that TSPO selectively protects GB cells against TRAIL-induced apoptosis hints to the possibility that TSPO may play an important role in immunotherapy resistance of GB. To further delineate the causes underlying the observed anti-apoptotic activity of TSPO, we conducted mRNA sequencing of TSPO proficient and deficient BTIC bulk cultures. In TSPO-deficient BTIC, a panel of significantly reduced genes was identified that can be associated with apoptosis-resistance of tumors and which may be crucial for mediating TSPO-driven GB immune resistance. Gene set enrichment analysis also revealed that TSPO modulates critical gene signatures in BTICs that are associated with stemness features of GB, allowing survival, self-renewing, adaption and differentiation into different lineages, and possibly inducing therapy-resistant mesenchymal characteristics.
GB is characterized by extensive tumoral heterogeneity and immense plasticity. Distinct degrees of TSPO-regulation in our BTIC-clones resulted in distinct responses to cytotoxicity and migration assays, which aligns well to data on the heterogeneity of GB in vitro, in animal models and in patients and which is also reflected by the heterogeneous response of patients with GB to targeted treatments.
In summary, we detected in the first funding period that pathways involved in proneural to mesenchymal transition are modulated by TSPO in BTIC. This transition is known to be modulated by tumor-intrinsic factors as well as the tumor microenvironment including the immune component adherent to GB. Our findings therefore raise the hypothesis that proneural to mesenchymal transition may be supported by IFN/TNF-mediated regulation of TSPO in the GB microenvironment. This will be addressed within the second funding period.
B1: Interaction between TSPO and TSPO ligands
Markus Zweckstetter, Göttingen
Nuclear magnetic resonance (NMR) spectroscopy of human TSPO
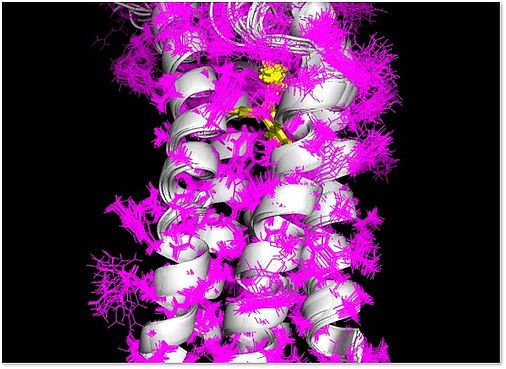
The three-dimensional structure of mouse TSPO (mTSPO) was previously determined by my lab using solution-state NMR spectroscopy. In addition, we studied the influence of a clinically relevant polymorphism in mTSPO, which results in the substitution of alanine by threonine at position 147 and decreases the affinities of many radioligands and therapeutics, on the mTSPO structure. We also found out that mTSPO is more flexible when no high-affinity radioligand is bound to the protein. A further focus of our studies was the analysis of the interaction of mTSPO with cholesterol using solid-state NMR spectroscopy. The three-dimensional structure of human TSPO is, however, unknown. In this project, we thus propose to study the structure of human TSPO (hTSPO) using solution- and solid-state NMR spectroscopy. To this end, we will first – using a model protein – perform tests on how to optimize the NMR process of resonance assignment and structure determination of alpha-helical membrane proteins. Subsequently, we will apply this methodology to hTSPO in the absence and presence of high-affinity ligands that are studied within the research unit (such as XBD173). This work will address the question if hTSPO folds – in the absence of hTSPO-specific ligands – into a stable conformation when it is solubilized in either detergents or lipids with distinct properties. Subsequent steps will be the determination of the structure of hTSPO, analysis of the molecular details of its interaction with selected high-affinity ligands and the influence of the A147T polymorphism on these interactions.
B2: TSPO in mitochondrial supercomplexes
Christine Ziegler, Christian Wetzel, Regensburg
Architecture and function of the mitochondrial VDAC/TSPO super-complex
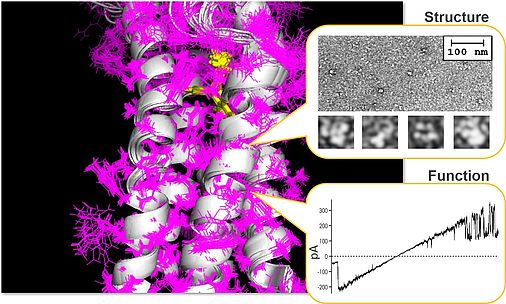
The translocator protein 18 kDa (TSPO) is a conserved outer mitochondrial membrane protein which is involved in the regulation of various aspects of mitochondrial and cellular physiology, including bioenergetics, lipid metabolism, cholesterol transport and steroid synthesis, as well as regulation of oxidative stress and Ca2+ homeostasis. Expression levels of the TSPO protein vary in different tissues and during various disease states such as neurodegenerative diseases, inflammatory processes and cancer, as well as neuropsychiatric disorders such as depression. These findings indicate that TSPO provides both general and specific functions related to cellular proliferation, survival and apoptosis, but also influences synaptic transmission and higher brain functions. To be able to fulfill these multifunctional tasks, TSPO has been proposed to be part of a multiprotein super-complex, thereby interacting with protein partners residing in the cytosol, outer mitochondrial membrane (OMM), intermembrane space, inner mitochondrial membrane, and the matrix. One of the best characterized effector proteins interacting with TSPO in the OMM is the voltage-dependent anion channel (VDAC), the major pore in the OMM, constituting the principle gateway for the transport and exchange of ions, ADP/ATP and metabolites. The mechanistic role of TSPO in mitochondrial function and its functional interaction with outer membrane proteins such as VDAC is largely unknown which is also due to missing structural data on the assembly and architecture of the proposed TSPO super-complexes.
Due to the need for structural information on TSPO-containing super-complexes in native membrane environment or artificial membrane mimics, and based on our own work on the impact of TSPO ligands on the multiple aspects of mitochondrial and cellular activity, the current project aims at (i) identifying the architecture of the mitochondrial VDAC/TSPO-containing super-complex by means of Cryo-EM single particle analysis, electron crystallography and tomography, (ii) the assembly of the super-complex together with additional proteins, e.g. hexokinase, GSK3beta, ATAD3A, and the ATP/ADP carrier, and (iii) at characterizing the modulatory capacity and the functional interaction within the VDAC/TSPO complex by means of biophysical and pharmacological characterization of VDAC-mediated currents in native and reconstituted protein complexes of defined composition and structure.
Our structural and functional studies will shed light on the complex interaction of TSPO and VDAC on a molecular level and will contribute to a deeper understanding of TSPO/VDAC-mediated signaling.
C1: Effects of TSPO ligands in the peripheral nervous system
Michael Schumacher, Paris
Neuroprotective and neuroregenerative effects of new TSPO ligands in the peripheral nervous system
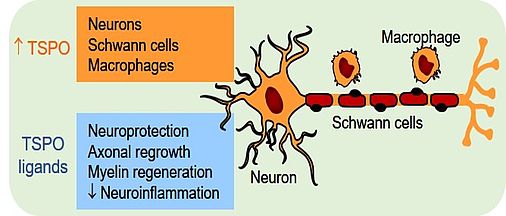
TSPO has been identified as an effective pharmacological target for protecting neurons and for increasing the speed of axonal regrowth in the peripheral nervous system. Agonistic ligands of TSPO therefore offer promising therapeutic perspectives for promoting peripheral nerve regeneration after traumatic injury or in patients with peripheral neuropathies. As part of a large mitochondrial membrane protein complex TSPO regulates many mitochondrial activities. Its best characterized function is the intramitochondrial transport of cholesterol, a rate-limiting step in steroid synthesis. However, the precise role of steroids in mediating the regenerative effects of TSPO ligands remains to be determined.
Grünenthal has developed new potent TSPO activators, which improve functional regeneration of both sensory neurons and motoneurons and prevent the development of neuropathic pain after severe peripheral nerve injury. These particularly efficient compounds are in fact dual-target ligands, as they activate both TSPO and voltage-gated delayed-rectifier potassium channels of the Kv7 family. These ion channels are involved in the regulation of neuronal excitability and play a role in the development of neuropathic pain. This has led to the hypothesis that the co-activation of both TSPO and Kv7 channels may be additive, or even reinforce the pharmacological effects of the individual mechanisms.
Our objectives are to experimentally test this dual-activation hypothesis and to clarify the role of steroids in the neuroprotective and neuroregenerative efficacy of TSPO activation. To reach these aims we have set up experimental rat models of severe crush injury of cervical spine nerves and of the sciatic nerve. Moreover, we will use transgenic, pharmacological and biochemical tools. The respective contribution of TSPO and Kv7 channel activation in the curative potential of the Grünenthal compounds can be investigated due to the advent of TSPO knockout rats and the availability of selective TSPO and Kv7 ligands. The importance of TSPO-stimulated steroid synthesis will be studied by using selective and potent inhibitors of steroidogenic enzymes and steroid receptors. Changes in peripheral nerve steroid levels will be analyzed by gas chromatography-tandem mass spectrometry (GC-MS/MS). This technology has become a reference method for the specific, sensitive and robust analysis of steroids in small biological samples. Our GC-MS/MS platform will therefore become a centralized facility for steroid analysis for the whole research unit.
Promoting neuroprotection and neuroregeneration corresponds to an unmet medial need. The delineation of TSPO in combination with Kv7 channel activation may constitute a novel therapeutic approach for peripheral nerve lesions and for peripheral axonopathies, including diabetic and toxic neuropathies, which all are characterized by a reduced regenerative capacity.
C2: Effects of TSPO ligands in retinal degeneration
Antje Grosche (née Wurm), Munich
In-depth in vivo analysis of TSPO functions in retinal degeneration
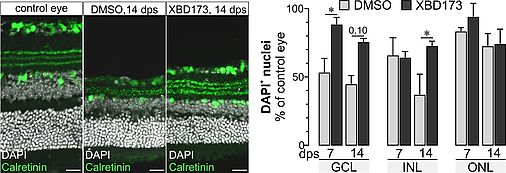
Retinal degeneration goes along with the activation of microglia (retinal macrophages) and with gliotic changes of the major retinal macroglia, the Müller cells. We have shown that Müller cell gliosis leads to a loss of their homeostatic functions with consequences for the survival of retinal neurons. Stimulating neurosteroid signaling, however, partially restored Müller cell functions. Recent reports on the regenerative potential of TSPO ligands indicate that part of their effects may be due to an enhanced mitochondrial neurosteroid production and an altered activation pattern of macro- and microglial cells. In our pilot study we tested the effects of the TSPO ligand XBD173 as a treatment option in a model of retinal ischemia/reperfusion. Retinae of mice treated with XBD173 showed an altered glial response pattern in view of a concomitantly improved maintenance of their neuron-supportive homeostatic functions. We also observed that XBD173 treatment reduced loss of neurons in postischemic retinae. Given that cell type-specific expression analysis demonstrated high TSPO expression in retinal pigment epithelial, endothelial and Müller cells, moderate expression in microglia, but no expression in neurons, this implies that stimulation of TSPO activity via XBD173 modulates processes of tissue protection or repair acting on non-neuronal cells and, more specifically, primarily on glial cells.
The present project aims to characterize the effects of TSPO ligands on glial cell functions in retinal disease with the following objectives: First, we will transfer our pilot experiment on retinal ischemia to another agonistic TSPO ligand (GRT16) to identify the compound targeting TSPO with highest neuroprotective potential in the retina. Second, generating and using microglia- and Müller cell-specific TSPO knockout mice we aim to determine which glial cell type (micro- or Müller glia) mediates the protective effects of the TPSO agonists in the postischemic retina. Next, to get insight into the impact of TSPO on retinal steroid biosynthesis, we will perform steroid profiling on retinae of healthy and in postischemic retinae of wild type as well as of micro- and Müller glia-specific TSPO knockout mice with or without treatment with TSPO ligands. In parallel, we will investigate the expression of genes involved in neurosteroid synthesis in the respective TSPO knockout mice. Finally, aiming to shed light on TSPO functions in CNS macroglia, we seek to unravel underlying TSPO related mechanisms by investigating mitochondrial integrity and energy status of wild type and TSPO-deficient Müller glia from the healthy and postischemic retina in absence and presence of TSPO ligands.
In conclusion, our project may help to evaluate the therapeutic potential of TSPO ligands to treat retinal diseases.
C3: Effects of TSPO ligands on brain plasticity
Mario Dorostkar, Jochen Herms, Munich
The role of TSPO on dendritic spine structural plasticity in health and neurodegenerative diseases
Microglia and proteins known as mediators of inflammation, e.g. complement cascade components, are involved in the physiological removal of synapses, a process termed synaptic pruning. This process occurs as part of reshaping of synapses during adolescence as well as during learning and memory. Moreover, activation of microglia and inflammatory alterations in the central nervous system are prominent features of many neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. We hypothesize that chronic microglia activation in these conditions causes pathological loss of synapses due to aberrant activation of these physiological mechanisms. Similar mechanisms resulting in brain-region specific synapse loss during adolescence are suggested to occur in psychoses.
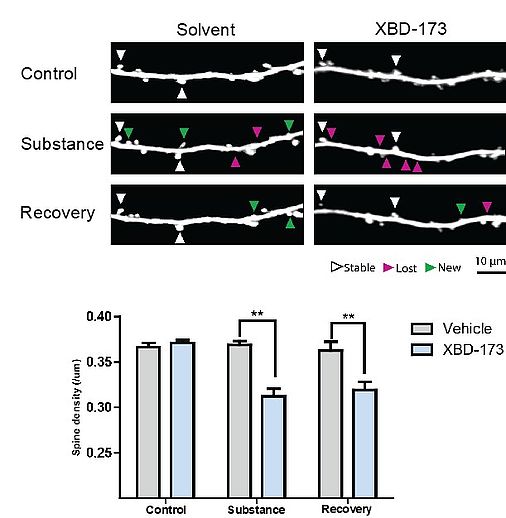
Increased microglial expression of TSPO is a typical hallmark of brain inflammation, which has been found also in neurodegenerative diseases. In preliminary experiments we showed that activation of TSPO leads to a reduction of excitatory postsynapses even in the absence of inflammation. Furthermore, pharmacological modulation of TSPO activity in a mouse model of Parkinson’s disease abolished the loss of cortical excitatory postsynapses.
As first part of this project we plan to study the effect of TSPO ligands and TSPO activity on synapse density and turnover under physiological conditions using in vivo multiphoton imaging and ex vivo confocal imaging. We will use microglia-specific TSPO knockout animals to understand the role of microglia activity and microglial TSPO in physiological synapse turnover. Subsequently, we plan to study the effects of microglial TSPO knockout and pharmacological modulation of TSPO activity in animal models of neurodegenerative diseases. By these means we will test whether TSPO constitutes a promising drug target for the treatment of synapse loss, which represents the structural correlate of cognitive decline in neurodegenerative diseases. In collaboration with project C4 we will further study the effects of TSPO modulation in animal models of stress and anxiety on synaptic plasticity.
Finally, we aim to study the activation states of microglia in treated and untreated animal models of Alzheimer’s and Parkinson’s disease using RNASeq on isolated microglia. These results may reveal further details on the cellular mechanisms of microglia activation and novel pathways, which may be targeted by pharmacological treatment in these diseases.
C4: Effects of TSPO ligands on fear, anxiety and emotionality
Inga D. Neumann, Oliver J. Bosch, Regensburg
Neurobiological analysis of behavioral effects of TSPO ligands in animal models of stress, anxiety and fear
 TSPO ligands may represent potential novel therapeutic agents for stress related and anxiety disorders. TSPO initiates neurosteroidogenesis in mitochondria, and neurosteroids in turn modulate GABAA receptor functions, which regulate stress reactivity and emotionality. TSPO ligands have been suggested to exert anxiolytic effects even with a more favorable side effect profile than benzodiazepines. However, underlying neurobiological mechanisms of their anxiolytic effects or target brain regions are mostly unknown, due to a lack of studies performed in respective animal models of anxiety, social anxiety or depression.
TSPO ligands may represent potential novel therapeutic agents for stress related and anxiety disorders. TSPO initiates neurosteroidogenesis in mitochondria, and neurosteroids in turn modulate GABAA receptor functions, which regulate stress reactivity and emotionality. TSPO ligands have been suggested to exert anxiolytic effects even with a more favorable side effect profile than benzodiazepines. However, underlying neurobiological mechanisms of their anxiolytic effects or target brain regions are mostly unknown, due to a lack of studies performed in respective animal models of anxiety, social anxiety or depression.
Our laboratory studies the neurobiological basis of emotional dysregulations using a well-characterized and pharmacologically validated animal model of pathological emotionality, i.e. Wistar rats selectively bred for high (HAB) and low (LAB) anxiety-related behavior. These represent a valuable tool for testing anxiolytic and antidepressive compounds. Moreover, we have recently established a mouse model specifically for social fear (social fear-conditioning paradigm). In both rodent models, we could show the selective and brain region-specific contribution of the neuropeptides oxytocin and vasopressin to the expression of anxiety-related behavior and social fear.
In this project C4, we aim to compare TSPO expression in distinct brain regions of HAB/LAB rats in comparison with non-selected animals using PET imaging (with project A1). Furthermore, we will test the effects of selected TSPO ligands (etifoxine, GRT16) in comparison to the benzodiazepine alprazolam on anxiety-related and depression-like behavior, and stress reactivity in HAB/LAB rats. The effectiveness of these TSPO ligands will also be studied in mice in the context of social versus non-social fear, and TSPO knockout mice. The ligands will be applied either into the brain ventricle system or into selected brain regions. Moreover, we will investigate the possible involvement of oxytocin and vasopressin in the observed TSPO effects. We will also quantify neurosteroid levels (with project C1) and spine density (with project C3) in brain regions relevant for anxiety and fear in HAB/LAB rats after treatment with TSPO ligands. Finally, as TSPO expression is found in bacteria, and bacterial TSPO shows similarities to mammalian TSPO, we aim to study the effects of TSPO ligands on microbiome composition in rats (with project C5). Some experiments will be performed also in females to address sex-specific effects.
Our results will help to identify target brain regions for TPSO ligands as well as differences in treatment efficacy and side effect profiles of TSPO ligands versus benzodiazepines using appropriate animal models. These findings will be of value for the translation to humans in project C5.
C5: Effects of TSPO ligands on fear and anxiety-related brain networks in humans
Caroline Nothdurfter, Jens Schwarzbach, Rainer Rupprecht, Regensburg
Differential effects of TSPO ligands on functional connectivity and metabolism related to fear and anxiety in the human brain
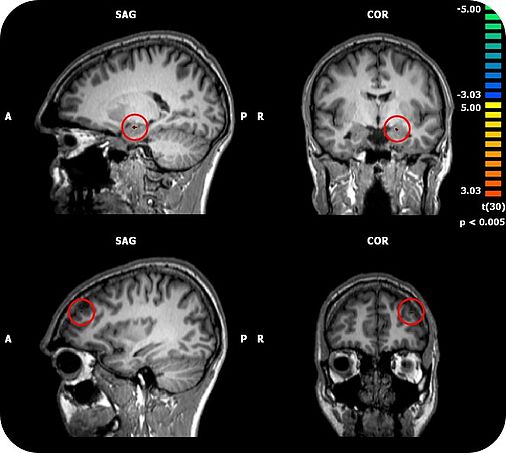
TSPO has been shown to be involved in the pathophysiology of depression and anxiety disorders. There is first evidence that TSPO ligands may exert anxiolytic properties. A major challenge for the development of novel anxiolytics is to identify compounds with a fast onset of action, which, in contrast to benzodiazepines, lack tolerance development and abuse liability. Our group has shown that the TSPO ligand XBD173 enhanced GABAergic neurotransmission in brain slices via the induction of neurosteroidogenesis and effectively reduced the number of pharmacologically induced panic attacks in rodents in the absence of sedation.. Importantly, XBD173 displayed anti-panic and anxiolytic efficacy in humans using an experimental anxiety paradigm. Whereas the benzodiazepine alprazolam caused sedation and withdrawal symptoms after only 7 days of treatment, these were absent in the XBD173 treated subjects. Moreover, repeated administration of XBD173 induced neither tolerance to its anxiolytic-like effects nor withdrawal symptoms in rodents. Thus, TSPO represents a promising target for the development of fast-acting anxiolytics with a more favorable side effect profile than benzodiazepines. Using brain stimulation, metabolic and functional brain imaging in this project we will therefore investigate how TSPO modulates neuronal networks related to fear and anxiety and to what degree such neuronal networks are related to the inherent efficacy/side effect profile of TSPO ligands compared to benzodiazepines. Since there is upcoming evidence that psychotropic drugs may affect human microbiome composition and bacteria also express TSPO, it is also of interest to study the effects of TSPO ligands on microbiome composition in relation to behavioral and neuroimaging parameters.
To address these issues we will perform a placebo-controlled clinical study in healthy male volunteers using a repeated measures design in which 36 participants sequentially will receive three different treatments (alprazolam 1.5 mg/day, etifoxine 150 mg/day, placebo) for 5 days followed by a 7 days washout in a randomized order. We will use an emotional paradigm which allows distinguishing between fear and anxiety at baseline and after each treatment condition combined with neuroimaging. Neuronal networks will be studied by means of resting state and task based functional magnetic resonance imaging. Brain metabolites will be studied by means of magnetic resonance spectroscopy. Moreover, we will assess physiological and neuroendocrine parameters, intra- and subcortical inhibitory function by paired pulse transcranial magnetic stimulation, and microbiome composition.
The respective findings may help to explain the observed differences in the side effect profile between TSPO ligands and benzodiazepines. This should help to identify neuronal networks and pathways which should be targeted for therapeutic reasons to avoid tolerance development and abuse liability for novel anxiolytic compounds.